Device Experience
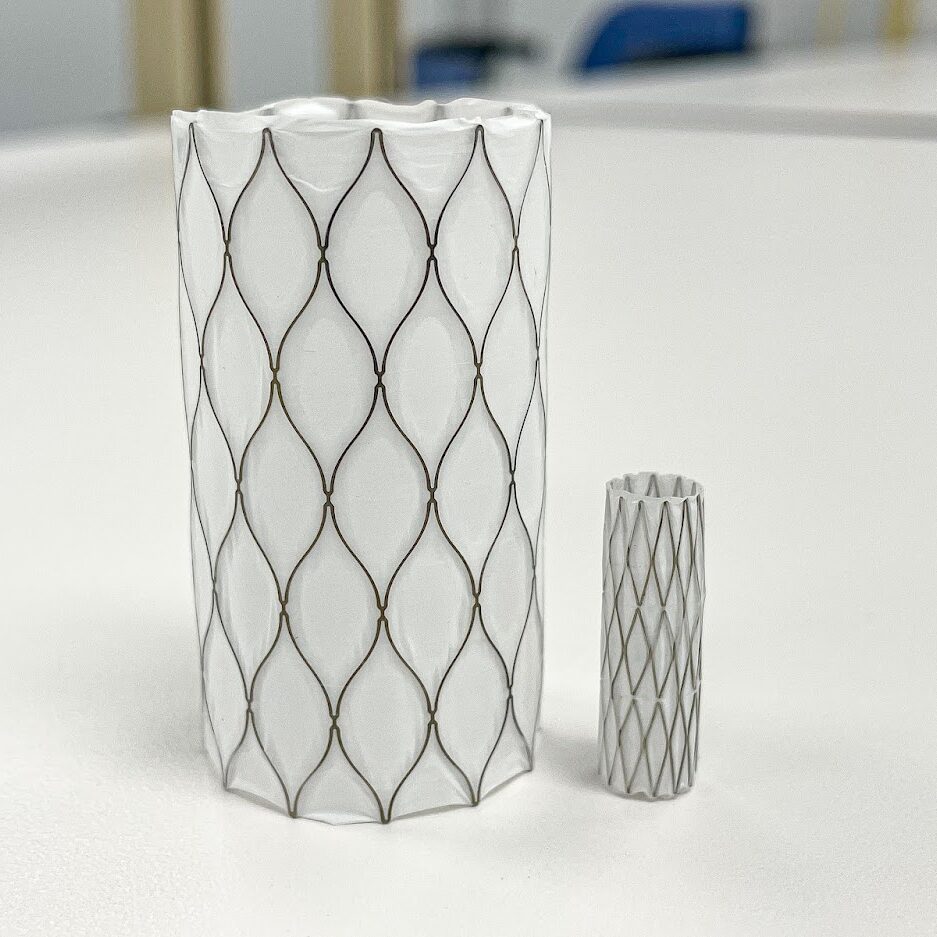
Medical Murray's experience with the Aortic and Endovascular device market allows us to be skilled in the design and manufacturing of both implants and their delivery systems. Most often, these are catheter delivery systems inserting a stent-graft into the Aorta to repair an aneurysm. Our experience in developing and manufacturing Aortic devices includes the following:
Abdominal Aortic Aneurysm (AAA) Stent Grafts & Delivery Systems
Thoracic Aortic Aneurysm (TAA) Stent Grafts & Delivery Systems
Aortic Dissection Repair Devices
Occlusion Catheters
Embolic Deflection & Embolic Filter Devices
Device Experience
Medical Murray's experience with the Aortic and Endovascular device market allows us to be skilled in the design and manufacturing of both implants and their delivery systems. Most often, these are catheter delivery systems inserting a stent-graft into the Aorta to repair an aneurysm. Our experience in developing and manufacturing Aortic devices includes the following:
Abdominal Aortic Aneurysm (AAA) Stent Grafts & Delivery Systems
Thoracic Aortic Aneurysm (TAA) Stent Grafts & Delivery Systems
Aortic Dissection Repair Devices
Occlusion Catheters
Embolic Deflection & Embolic Filter Devices
Materials & Production
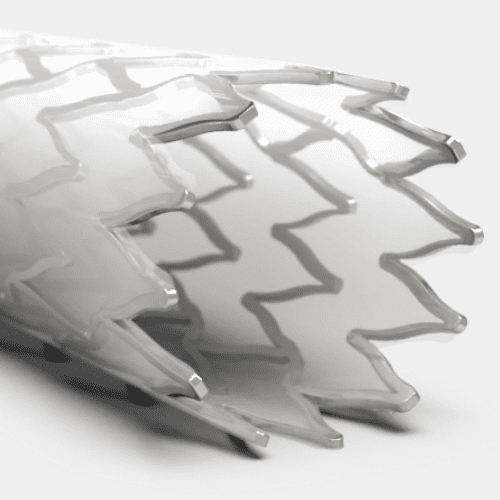
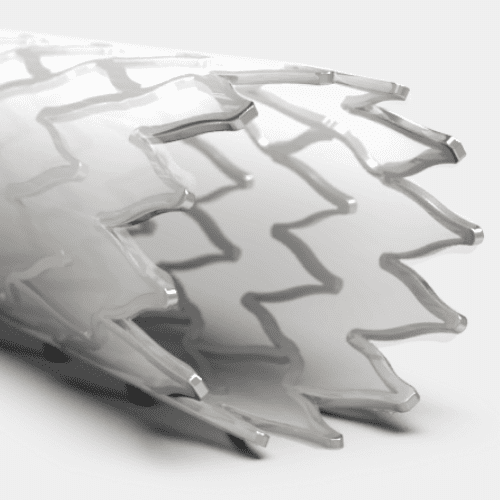
By utilizing our in-house cleanroom production environment, we can ensure the implant and the delivery system match stringent cleanroom manufacturing requirements.
Medical Murray can also perform the assembly operations your device needs for production, such as laser cutting or heat setting stents, or sewing and sintering graft material onto the stent frame. We can also support the crimping and loading of the implant into its delivery system, and the packaging & sterilization services for the finished device.
Graft Materials
ePTFE
Polyester
Polyurethanes
Stent Frame Materials
Nitinol
Stainless Steel
Bioabsorbables/Bioresorbables
Materials & Production
By utilizing our in-house cleanroom production environment, we can ensure the implant and the delivery system match stringent cleanroom manufacturing requirements.
Medical Murray can also perform the assembly operations your device needs for production, such as laser cutting or heat setting stents, or sewing and sintering graft material onto the stent frame. We can also support the crimping and loading of the implant into its delivery system, and the packaging & sterilization services for the finished device.
Graft Materials
ePTFE
Polyester
Polyurethanes
Stent Frame Materials
Nitinol
Stainless Steel
Bioabsorbables/Bioresorbables