At Medical Murray, we’ve seen how minimally invasive surgery (MIS) has continued to revolutionize patient care over the past 3 decades. By reducing recovery times, minimizing scarring, and lowering the risk of complications, MIS is reshaping the medical field. A critical component of this transformation is the development of sophisticated implant delivery systems that allow for precise placement of medical devices through small incisions or natural openings.
We’d like to share our insights into the primary types of implant delivery systems used in MIS, specifically catheter-based, endoscopic, and robotic-assisted systems. We will also discuss the key technical considerations essential for their development.
Understanding Minimally Invasive Surgery and Implant Delivery Systems
MIS techniques aim to limit the size and number of incisions needed for surgical procedures. The success of these techniques heavily relies on the implant delivery systems that transport and deploy devices within the body with high precision. These systems must navigate complex anatomical structures while maintaining the integrity and functionality of the implants they deliver.
Key Types of Implant Delivery Systems
Catheter-Based Implant Delivery System

Catheter-based systems are flexible tubes inserted into the body’s vascular or luminal structures, often guided by real-time imaging like fluoroscopy or ultrasound.
Applications:
- Cardiovascular Interventions: Delivering stents, stent grafts, and occlusion devices.
- Neurovascular Procedures: Placing coils or flow diverters for aneurysm treatment.
- Transcatheter Heart Implants: Deploying heart valve replacements and LAA implants.
Technical Considerations:
- Flexibility and Steerability: Utilizing materials like nitinol or polyurethane to navigate intricate anatomy.
- Hydrophilic Coatings: Applying coatings to reduce friction and improve maneuverability.
- Imaging Compatibility: Incorporating radiopaque markers for enhanced visibility during procedures.
Catheter-Based Implant Delivery System
Endoscopic systems involve instruments passed through endoscopes to deliver implants within hollow organs such as the gastrointestinal tract or respiratory system.
Applications:
- Gastrointestinal Procedures: Placing stents for esophageal strictures or biliary obstructions.
- Pulmonology: Deploying valves or coils in bronchoscopic lung volume reduction.
- Urology: Inserting ureteral stents or implants for urinary incontinence.
Technical Considerations:
- Articulation and Control: Designing devices with enhanced maneuverability to navigate complex pathways.
- Integration with Endoscopic Equipment: Ensuring compatibility with existing visualization tools.
- Biocompatibility and Sterilization: Selecting materials that withstand sterilization without degrading performance.
Robotic-Assisted Implant Delivery Systems
Robotic-assisted systems employ robotic platforms to enhance precision and control during implant delivery, often surpassing human limitations.
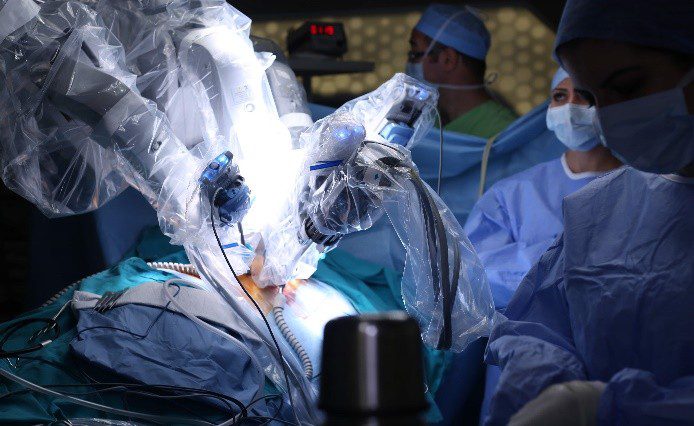
Applications:
- Cardiac Procedures: Robotic-assisted coronary artery bypass or valve repairs.
- Neurosurgery: Accurate delivery of deep brain stimulation electrodes.
- Orthopedic Surgery: Precise placement of joint prostheses or spinal implants.
Technical Considerations:
- Haptic Feedback: Providing tactile sensations to mimic manual procedures.
- Motion Scaling and Tremor Reduction: Implementing software that translates surgeon movements into precise actions.
- System Integration: Ensuring seamless communication between robotic components and imaging systems.
Other Implant Delivery Systems
Needle-Based and Laparoscopic Delivery Systems also play vital roles in certain MIS procedures.
Applications:
- Needle-Based Systems: Brachytherapy seed implantation, targeted drug delivery.
- Laparoscopic Systems: Mesh placement for hernia repair, organ resection assistance.
Technical Considerations:
- Access Limitations: Instruments are constrained by entry points and angles.
- Visualization: Often rely on external imaging or direct line-of-sight.
- Instrumentation: Balancing size with the need for rigidity and control.
Technical Considerations for Implant Delivery System Development
Developing advanced implant delivery systems isn’t just about innovation; it’s about understanding the practical challenges surgeons face and ensuring patient safety.
Material Selection
- Biocompatibility: We prioritize materials that won’t cause adverse reactions—like medical-grade stainless steel, titanium, nitinol, and biocompatible polymers.
- Mechanical Properties: Strength, flexibility, and fatigue resistance are crucial, especially for devices under stress.
- Sterilization Compatibility: Materials must withstand sterilization methods without degrading.
Design and Engineering
- Miniaturization: We’re constantly pushing the boundaries to make devices smaller without sacrificing function.
- Ergonomics: Devices should be intuitive, reducing fatigue and the potential for errors.
- Deployment Mechanisms: Innovative solutions enhance performance during implant deployment.
Self-Expanding Mechanisms
Self-expanding systems use materials like nitinol that expand to a predetermined shape upon deployment, eliminating the need for external expansion devices.
Key Factors for Self-Expanding Mechanisms:
- Material Properties: Utilizing shape-memory alloys that respond predictably.
- Controlled Deployment: Designing systems that keep the implant constrained until precise placement.
- Applications: Common in vascular stents and certain heart valves.
Balloon Expandable Mechanisms
Balloon expandable systems use a balloon catheter to expand the implant to its functional diameter at the target site.
Key Factors for Balloon Expandable Mechanisms:
Balloon expandable systems use a balloon catheter to expand the implant to its functional diameter at the target site.
- Precise Expansion: Balloons inflated to exact pressures achieve desired diameters.
- Material Compatibility: Implants must be malleable yet strong post-expansion.
- Applications: Often used in coronary artery stenting.
Comparing Self-Expanding and Balloon Expandable Systems:
- Deployment Control: Balloon systems offer immediate expansion; self-expanding devices rely on material properties.
- Radial Force: Balloon expandable stents provide higher initial force; self-expanding stents adapt better over time.
- Vessel Compatibility: Choice depends on vessel dynamics and specific clinical needs.
Loading the Implant
Properly loading the implant into the delivery system is crucial to prevent damage and ensure optimal performance.
Implant Loading Factors to Consider:
- Crimping Techniques: Uniform force application is essential when crimping implants like stents.
- Handling Procedures: Minimizing manipulation reduces contamination or deformation risks.
- Equipment Design: Utilizing specialized tools that align with the implant’s geometry and materials.
- Quality Assurance: Implementing inspections to detect any damage from the loading process.
Bioburden Management
When the implant is loaded into the delivery system, they become a single finished device, so controlling bioburden is essential.
Bioburden Management Factors to Consider:
- Sterilization Validation: Ensuring the combined device can be sterilized without compromising integrity.
- Packaging Design: Developing solutions that maintain sterility and protect the device.
- Cleanroom Manufacturing: Assembling in controlled environments to limit contamination.
- Regulatory Compliance: Adhering to standards like ISO 11737 for bioburden testing.

Manufacturing Processes
- Precision Machining: Techniques like CNC machining and laser cutting achieve tight tolerances.
- Surface Treatments: Coatings improve biocompatibility and may provide drug-eluting capabilities.
- Quality Control: Rigorous testing ensures device reliability.
Regulatory Considerations
- FDA Guidelines: Compliance ensures safety and efficacy. Understanding required submissions is essential.
- ISO Standards: Adherence to standards like ISO 13485 and ISO 10993.
- Documentation: Maintaining detailed records of design controls and risk assessments is crucial.
Testing and Validation
- Mechanical Testing: Assessing strength and deployment forces.
- Biological Evaluation: In vitro and in vivo studies for biocompatibility.
- Clinical Trials: Gathering data on safety and effectiveness.
Biodegradable Materials
Biodegradable systems use implants that gradually degrade, reducing the need for removal.
Biodegradable Material Factors to Consider:
- Material Degradation Rates: Matching breakdown timelines to healing processes.
- Mechanical Strength: Maintaining integrity throughout the implant’s lifespan.
- Biocompatibility: Ensuring degradation byproducts are non-toxic.
Applications:
- Drug-Eluting Stents: Localized drug delivery while the scaffold degrades.
- Orthopedic Implants: Biodegradable screws or plates.
- Tissue Engineering: Scaffolds that support regeneration before resorption.
Collaboration in Development
At Medical Murray, we believe collaboration is key. Developing advanced implant delivery systems benefits from multidisciplinary teamwork.

- Technical Expertise: Our specialists excel in device design and manufacturing, especially in catheter-based, endoscopic, and robotic-assisted systems.
- State-of-the-Art Facilities: We utilize advanced equipment for prototyping and manufacturing.
- Regulatory Support: We guide you through the complex regulatory landscape.
- Speed to Market: Our streamlined processes reduce development time.
Implant Delivery Systems: A Game-changer for MIS
The evolution of implant delivery systems is pivotal to advancing minimally invasive surgery. By focusing on catheter-based, endoscopic, and robotic-assisted delivery systems, we’re meeting the growing demand for precise, less invasive treatments.
Understanding the technical considerations—including deployment mechanisms, implant loading, and bioburden management—is essential. Through collaboration, we work with our partners to develop innovative solutions that enhance patient outcomes and drive the future of medical technology.
Interested in developing your next implant delivery system? Let’s work together. Contact Medical Murray to learn more!
