At Medical Murray, we know that developing a great transcatheter implant is only the first step. Once the design and materials are finalized, the next big challenge is manufacturing the device to meet the specifications required for safe and effective use. This is especially true for complex devices like covered stents and frames.
Here’s what we focus on to make sure we consistently deliver high-quality implants.
Keys to Effective Medical Implant Manufacturing
1. Precision Manufacturing and Tight Tolerances
The success of a transcatheter implant depends on its ability to perform exactly as designed, every time. That’s why precision is so important in the medical device manufacturing process. For covered stents and frames, tight tolerances are critical. Our advanced manufacturing technologies help us achieve the accuracy needed to ensure each implant functions properly in the body.
2. Consistent Quality Control
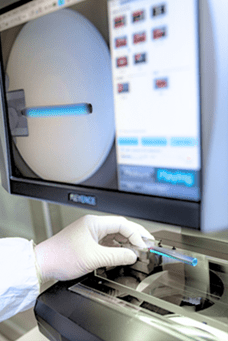
Consistency is key when you’re manufacturing medical devices that will be used in life-saving procedures. At Medical Murray, we take quality control seriously. From the moment production begins, we implement thorough testing and inspections. We use dimensional checks, fatigue tests, and other assessments to ensure that every implant meets the necessary standards before it’s shipped.
We also keep detailed records of each step in the medical device manufacturing process, so we can trace any issues back to the source if something doesn’t meet expectations.
3. Reliable Supply Chain
A reliable supply chain is crucial to making sure that every transcatheter implant meets the required specifications. We work closely with our suppliers to make sure that all materials, from metal frames to covering materials, are of the highest quality and are delivered on time. By managing our supply chain carefully, we avoid delays and ensure that production runs smoothly, even as demand increases.
4. Meeting Compliance Standards
Transcatheter implants are commonly classified as Class III medical devices, which means they must meet strict regulatory requirements to be approved for use. At Medical Murray, we follow ISO 13485 standards and meet FDA regulations throughout the manufacturing process. This helps us ensure that every implant we produce is safe, reliable, and ready for clinical use.

5. Scaling Up Medical Device Production
As demand grows, the ability to scale production while maintaining quality is a major focus. At our expanded manufacturing facilities, we have the space and technology needed to produce more implants without compromising on quality or delivery times. Our team works hard to make sure that, whether you need a small batch or large-scale production, we can deliver quality product on time and on spec.
6. Packaging and Sterilization

Once manufacturing is complete, the implant must be packaged and sterilized properly to ensure it remains sterile until it’s ready for use. We handle the packaging and post-sterilization release in-house, making sure the implants are safely sealed and ready to go to hospitals and clinicians without any issues.
Delivering Quality Medical Implant Manufacturing Every Time
At Medical Murray, we’re committed to making sure that every transcatheter implant we manufacture meets the highest standards. We focus on the details, from the supply chain to quality control, to make sure every product we ship is consistent, safe, and ready for clinical use.
If you’re looking for a manufacturing partner that can deliver reliable, high-quality transcatheter implants, contact us to learn how we can help.
